Vir Biotechnology (VIR.US) announced today that the US government will buy an additional 600,000 doses of sotrovimab, its Covid-19 treatment developed together with British partner GlaxoSmithKline (GSK.UK). The extra doses will be delivered in the first quarter of 2022 to the US, which has an option to purchase more in the second quarter.
Including today's announcement, the two companies have agreements for the sale of roughly 1.7 million doses of sotrovimab worldwide. Sotrovimab was granted emergency use authorization by the Food and Drug Administration in May 2021 for treatment of mild to moderate COVID-19 in at-risk adults and children 12 and older. Preclinical data has shown treatment is effective against all COVID-19 variants, including delta and omicron.
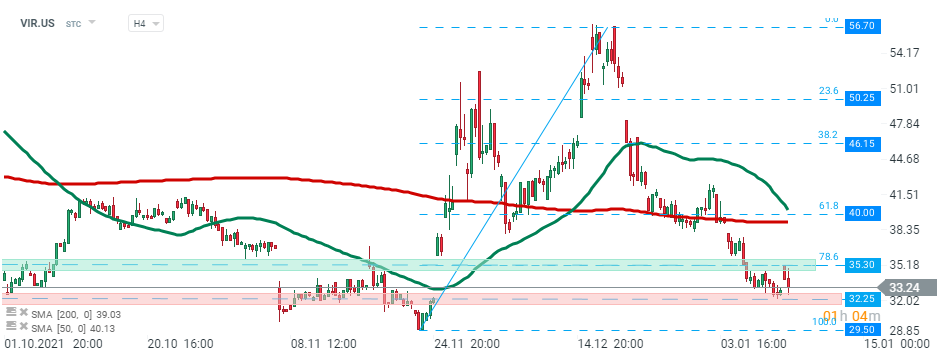 Vir Biotechnology (VIR.US) stock rose nearly 9.0% in premarket, however later in the session bullish momentum faded away. Buyers failed to break above the local resistance zone at $35.30 which is marked with 78.6% Fibonacci retracement of the last upward wave and price pulled back to recent lows at $32.25. Source: xStation5
Vir Biotechnology (VIR.US) stock rose nearly 9.0% in premarket, however later in the session bullish momentum faded away. Buyers failed to break above the local resistance zone at $35.30 which is marked with 78.6% Fibonacci retracement of the last upward wave and price pulled back to recent lows at $32.25. Source: xStation5

Kongsberg Gruppen after earnings: The company catches up with the sector

Market wrap: European indices attempt a rebound after Wall Street’s record selloff 🔨

Morning wrap: Tech sector sell-off (06.02.2026)

Amazon shares tumble 10% as investors recoil at the price of AI dominance
The content of this report has been created by XTB S.A., with its registered office in Warsaw, at Prosta 67, 00-838 Warsaw, Poland, (KRS number 0000217580) and supervised by Polish Supervision Authority ( No. DDM-M-4021-57-1/2005). This material is a marketing communication within the meaning of Art. 24 (3) of Directive 2014/65/EU of the European Parliament and of the Council of 15 May 2014 on markets in financial instruments and amending Directive 2002/92/EC and Directive 2011/61/EU (MiFID II). Marketing communication is not an investment recommendation or information recommending or suggesting an investment strategy within the meaning of Regulation (EU) No 596/2014 of the European Parliament and of the Council of 16 April 2014 on market abuse (market abuse regulation) and repealing Directive 2003/6/EC of the European Parliament and of the Council and Commission Directives 2003/124/EC, 2003/125/EC and 2004/72/EC and Commission Delegated Regulation (EU) 2016/958 of 9 March 2016 supplementing Regulation (EU) No 596/2014 of the European Parliament and of the Council with regard to regulatory technical standards for the technical arrangements for objective presentation of investment recommendations or other information recommending or suggesting an investment strategy and for disclosure of particular interests or indications of conflicts of interest or any other advice, including in the area of investment advisory, within the meaning of the Trading in Financial Instruments Act of 29 July 2005 (i.e. Journal of Laws 2019, item 875, as amended). The marketing communication is prepared with the highest diligence, objectivity, presents the facts known to the author on the date of preparation and is devoid of any evaluation elements. The marketing communication is prepared without considering the client’s needs, his individual financial situation and does not present any investment strategy in any way. The marketing communication does not constitute an offer of sale, offering, subscription, invitation to purchase, advertisement or promotion of any financial instruments. XTB S.A. is not liable for any client’s actions or omissions, in particular for the acquisition or disposal of financial instruments, undertaken on the basis of the information contained in this marketing communication. In the event that the marketing communication contains any information about any results regarding the financial instruments indicated therein, these do not constitute any guarantee or forecast regarding the future results.


