Pfizer (PFE.US) completed clinical trials for a drug called Paxlovid, which aims to reduce the risk of severe coronavirus infection. The researchers announced that their tests suggested that taking Paxlovid orally reduces the risk of severe disease or even death by almost 89%.
Paxlovid refers to the overall treatment, which consists of taking ritonavir, an HIV antiviral drug, and new molecules created by scientists at Pfizer that were originally intended to counteract SARS infection nearly two decades ago. The medicine is in the form of three tablets taken twice a day. Company assured that test results will be immediately sent to the Food and Drug Administration (FDA), which is to approve the use of the drug in life-threatening situations.
"These data suggest that our oral antiviral candidate, if approved by regulatory authorities, has the potential to save patients' lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations,'' Pfizer CEO Albert Bourla said in a statement. The reported effectiveness of the drug is therefore greater compared to the drug molnupiravir from Merck & Co Inc., which reduces the risk by half (preliminary information).
The company plans to produce 180,000 packages of the drug by the end of the year and at least 50 million by the end of 2022. Experts also remind that vaccines are still the most effective way to protect against COVID.
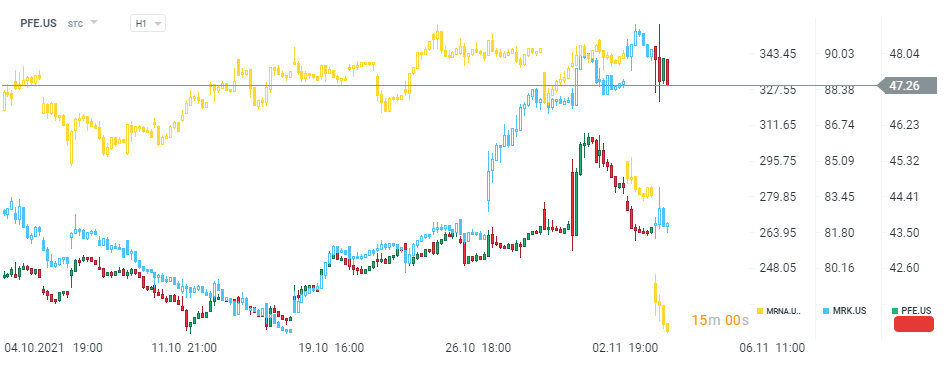 Pfizer's stock (PFE.US) launched today's session with a massive bullish price gap, while shares of its main competitors Merck MRK.US) and Moderna (MRNA.US) plunged. Source:xStation5
Pfizer's stock (PFE.US) launched today's session with a massive bullish price gap, while shares of its main competitors Merck MRK.US) and Moderna (MRNA.US) plunged. Source:xStation5

Daily summary: Silver plunges 9% 🚨Indices, crypto and precious metals under pressure

Does the current sell-off signal the end of quantum companies?

Howmet Aerospace surges 10% after earnings reaching $100 bilion market cap 📈

US Open: Cisco Systems slides 10% after earnings 📉 Mixed sentiments on Wall Street


