The biotech company Novavax (NVAX.US) has risen more than 20% during today's trading session on Wall Street, following news from the head of the European Medicines Agency (EMA). As reported by Reuters, the head of the EMA said that a vaccine being developed by US company Novavax will be authorized "in the very near future". The company's vaccine has so far been authorized in Indonesia and the Philippines. During clinical trials in the US, UK and Mexico, the NVX-CoV2373 vaccine has demonstrated over 90% efficacy. The company announced on 2 December that it is able to produce Omicron variant vaccines as early as January 2022.
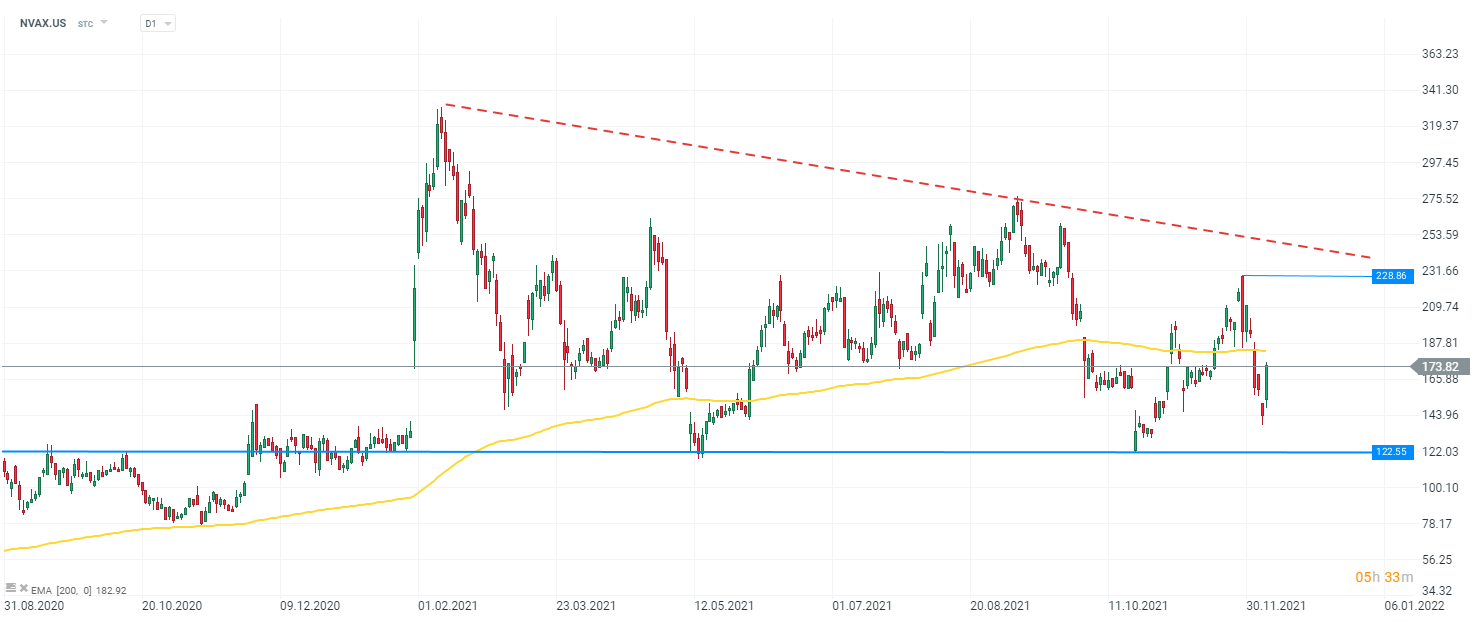
Novavax (NVAX.US) stock, D1 interval. From the perspective of technical analysis, the company is moving in a descending triangle formation. Currently, the price is testing the resistance set by the 200 EMA (yellow line). If the demand side manages to break above this limit, it will be possible to test the local peak near $229 per share. If the upside is stopped, the key support remains the lower limit of the descending triangle formation at $122.5 usd per share. Source: xStation 5

Daily summary: Silver plunges 9% 🚨Indices, crypto and precious metals under pressure

Does the current sell-off signal the end of quantum companies?

Howmet Aerospace surges 10% after earnings reaching $100 bilion market cap 📈

US Open: Cisco Systems slides 10% after earnings 📉 Mixed sentiments on Wall Street


