Sanofi shares came under significant pressure following the release of negative news regarding tolebrutinib, an innovative drug being developed for the treatment of multiple sclerosis. The company announced that the U.S. Food and Drug Administration will not issue a regulatory decision by the originally planned date of December 28, 2025. A new timeline has not yet been set, and Sanofi expects further guidance from the FDA only by the end of the first quarter of 2026. At the same time, at the regulator’s request, a program has been launched that will allow some patients to access the therapy before the official decision.
The company’s situation was further worsened by clinical trial results. In a large Phase 3 study, tolebrutinib did not achieve the expected outcomes for patients with primary progressive multiple sclerosis. The drug did not slow disease progression compared to placebo, leading Sanofi to abandon plans to seek approval for this indication, which affects roughly ten percent of people with multiple sclerosis.
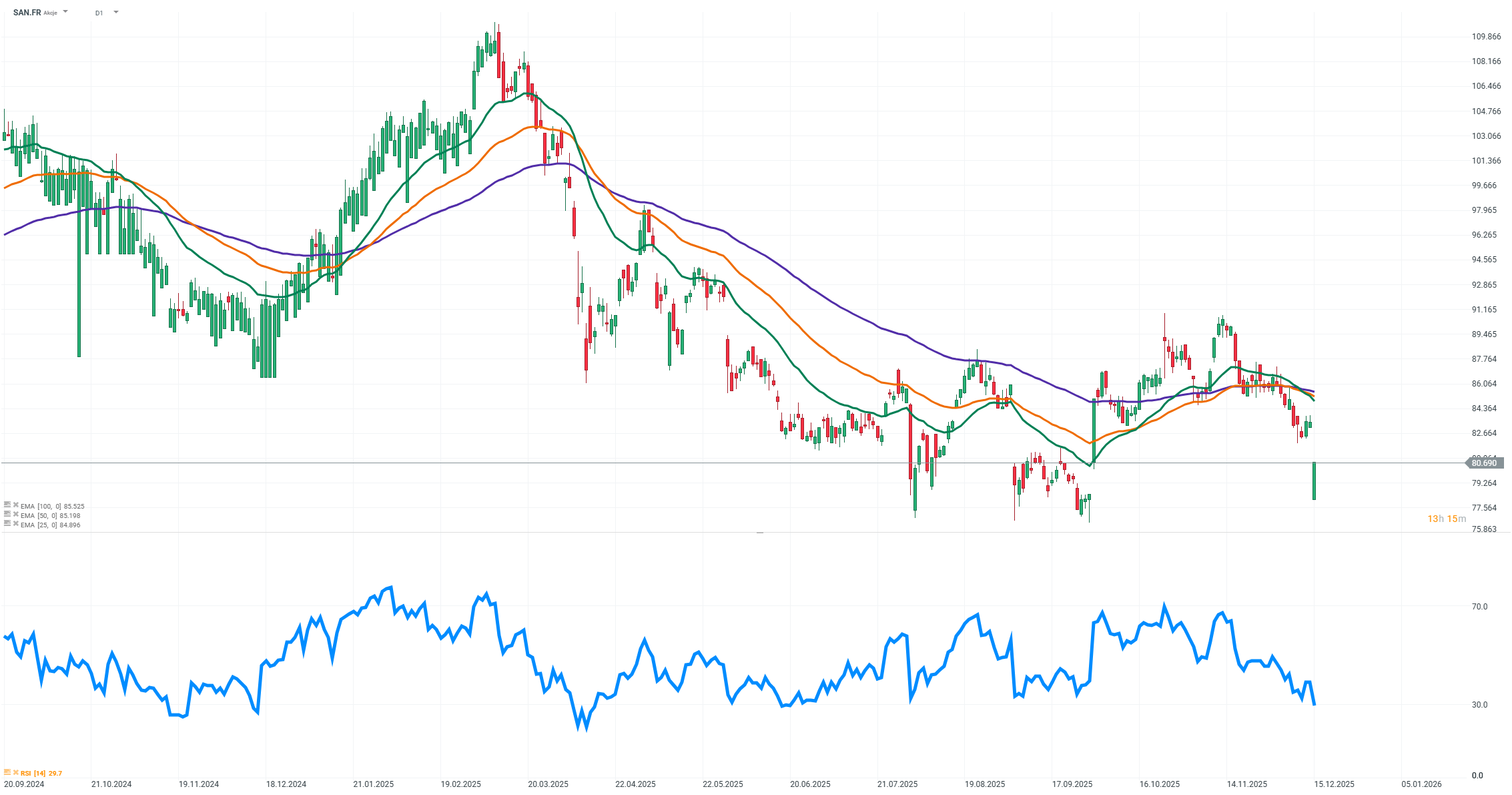
In response, the company announced it will conduct an impairment test for the tolebrutinib project, with results expected in January. At the same time, Sanofi reaffirmed its financial guidance for 2025 and emphasized that it still believes in the drug’s potential in its main indication. However, the market reacted nervously. The company’s shares on the Paris exchange fell by more than 4%, marking the largest one-day decline in three months. Markets are beginning to question previous peak sales estimates of around 1.7 billion dollars per year, although some analysts still see value in the project’s key indication.
Source: xStation5

Daily summary: Indices and crypto decline amid rising oil prices 🚩 Gold and the US dollar move higher

Boeing gains amid news about potential huge 737 MAX order from China 📈

Oil surges 11% amid escalating Middle-East conflict 📈VIX gains driven by fear on Wall Street

Bitcoin loses the momentum again 📉Ethereum slides 5%


